The separation of patient profile data from clinical trial data in clinical data management systems (CDMS) is essential for maintaining data privacy, regulatory compliance, and facilitating independent analysis of patient information.
This approach aligns with the Health Insurance Portability and Accountability Act (HIPAA) principles, particularly the “minimum necessary” access to protected health information (PHI).
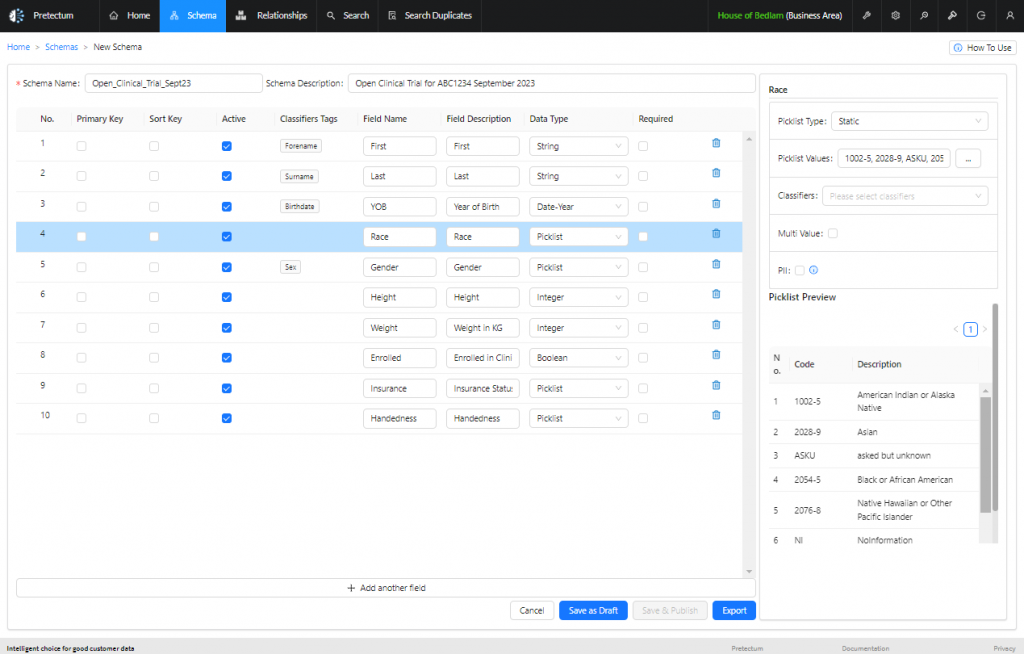
Vital Information
Patient profiles typically encompass vital information, including demographics, medical history, and treatment exposure.
By storing basic patient identifiers (such as name, date of birth, and contact information) separately from sensitive medical details, the risk of unauthorized access or accidental disclosure of PHI is significantly reduced.
Compartmentalization enhances data privacy and safety outcomes, making it more challenging to re-identify individuals in the event of a data breach. This is where a platform like the Pretectum CMDM can be of assistance.
HIPAA mandates the separation of PHI from non-PHI data, although it does not explicitly require encryption of PHI. Instead, encryption is categorized as an “addressable” implementation specification within the Security Rule. Other regulations like HITRUST, ISO-27001, GDPR and SOC-2 often apply but specifically in the way the data is stored and shared in order to protect sensitive data.
While not mandatory, encryption is highly recommended to protect electronic PHI (ePHI).
The separation of patient profiles from trial data not only aids in compliance but also fosters patient trust. Patients are more likely to participate in clinical trials if they believe their personal information is handled with care and separated from sensitive medical data. Pretectum CMDM stores all data at rest in an encrypted store bound up by complex and a fine-grained permission structure under a Role Based Authorization Concept (RBAC).
The Clinical Data Interchange Standards Consortium (CDISC) has established standardized formats for clinical research data, which are crucial for regulatory submissions, particularly to the U.S. Food and Drug Administration (FDA). The Study Data Tabulation Model (SDTM) is a foundational standard that organizes clinical trial data into structured formats, allowing for efficient data sharing and analysis across different studies.
Key Domains: Demographics (DM) and Subject Characteristics (SC)
- Demographics (DM) Domain: This domain captures essential demographic information such as unique subject identifiers, age, sex, race, ethnicity, and country of residence. This data is collected once at the trial’s outset, ensuring consistency and integrity for demographic analysis.
- Subject Characteristics (SC) Domain: This domain provides additional characteristics of subjects not captured in the DM domain, such as education level and marital status. Similar to the DM domain, SC data is collected once, maintaining stability for analysis.
By utilizing standardized formats, researchers can conduct robust analyses regarding the influence of demographic factors on trial outcomes, thereby enhancing the transparency and efficiency of clinical research.
The independent management of patient profiles supports data integrity and facilitates easier updates, particularly in longitudinal studies where participants may be involved in multiple trials over time.
To learn more about how you could use the Pretectum CMDM to secure your patient and participant data contact us today.


