Why Your Customer Data Is Your Greatest Risk—And Your Greatest Asset
These days trust is the ultimate currency. Commercial banks, insurance companies, and investment firms are built on a foundation of security, reliability, and fiduciary responsibility.
Yet, a stark and unsettling statistic has emerged as a major fault line in this foundation: per Statista, over 737 reported data compromises have targeted financial institutions in 2024 alone, a number that continues to climb.
For an industry that invests billions in cyber defenses, regulatory compliance, and proprietary banking systems, this should be a wake-up call. And the problem isn’t always the external hacker; more often, it’s internal chaos, lax controls, weak systems and the human element.
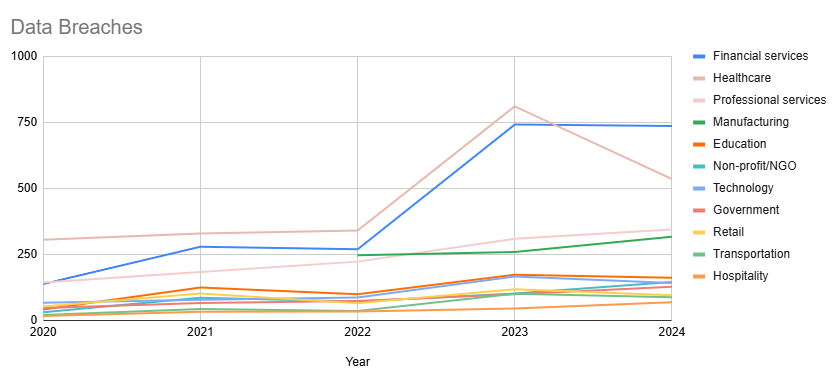
Customer data gets fragmented across a labyrinth of legacy and modern systems, departmental silos, and third-party vendors are also part of the liability. It’s not a single vulnerability but a systemic weakness, the proverbial death of a thousand small papercuts that represent the gaps in the armor that bad actors exploit as part of any given breach.
And the truth is, that many financial institutions are managing the concept of a “customer” that doesn’t actually exist. With collections of partial, conflicting, and outdated records all living independently in an array of core banking systems, CRM platforms, loan origination software, and myriad other applications. These are far from golden customer records; they’re shameful fragmented ghosts of a single customer at a point in time, each telling a potentially different story. Does this all sound familiar?
The Cost of Data Silos
Fragmentation and data silos doesn’t represent merely an inconvenience; it’s is a clear, present and direct threat to the bottom line and the brand.
Risk and Compliance – Privacy and personal data handling regulations and evolving KYC (Know Your Customer) and AML (Anti-Money Laundering) measures demand a single, verifiable source of truth for every customer. When the customer data is inconsistent across systems, it creates significant risk vectors to the institution and makes it nearly impossible to demonstrate compliance with confidence. Audits become a manual, time-consuming nightmare, and the specter of massive fines looms large.
Operational Inefficiency – So, consider the simple act of a customer updating their address. Does that change cascade across all systems in real-time? Or does it require a manual ticket, a phone call, and a series of data entry tasks that inevitably lead to errors? This constant, costly effort to reconcile data across departments bleeds resources and slows down critical business processes.
Customer Experience – Today’s customer expects a seamless, personalized experience. Consumers don’t care that your mortgage department can’t see their investment history or that your call center agent doesn’t have the full picture of their account activity. Disjointed interactions and a lack of contextual understanding erode trust and drive customers toward more agile, data-savvy competitors.
The Promise of Customer Master Data Management (CMDM)
For quite some time now, the solution has been to build more complex integrations, creating a tangled web of point-to-point connections that are brittle and difficult to maintain. Such a “spaghetti architecture” only compounds the problem.
A new approach is needed, one that doesn’t just manage data but masters it.
This is the core mission of Pretectum CMDM. We recognize that for heritage financial institutions, deploying a “rip and replace” approach to customer data management is not a viable strategy. Your core banking systems are the engines of your business, built over decades with enormous investment. Our solution is designed to work with, not against, your existing infrastructure.
How Pretectum CMDM Reimagines Financial Data Management
Pretectum is not a generic data platform like a Salesforce, SAP or Informatica system, nor is it a purely marketing-focused CDP. We are purpose-built Customer MDM (cMDM) for the unique challenges of customer master data that can be used in a highly regulated industry. Our solution offers a new way of thinking about your likely most valuable data asset – your customer data profile.
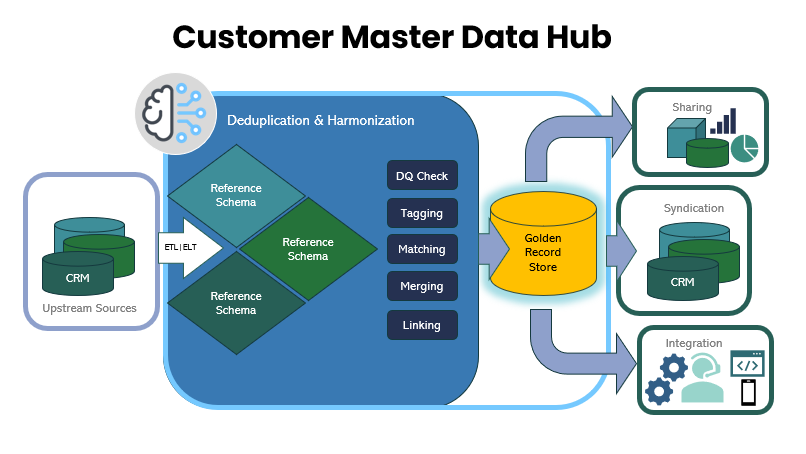
Federated Architecture for the Real World– We don’t ask you to migrate all your data. Our federated approach allows us to connect to your proprietary systems, market-leading commercial offerings, and other data sources where they live. We pull in data, cleanse and enrich it, and create a single, authoritative “golden record” for each customer. This single source of truth is then syndicated back to your operational systems, ensuring consistency without a massive, disruptive data migration project.
Real-Time Data Governance and Quality – In financial services, just a single inaccuracy can have cascading and catastrophic consequences; so we move beyond periodic batch cleansing. Instead we includes real-time data entry validation and scheduled and on-demand AI-based quality inspections. This means that a new customer record is checked for accuracy and completeness the moment it’s created, preventing bad data from ever entering your ecosystem.
Empowering the Customer with Self-Service– The best data is often the data verified by the source and here, our unique customer self-service feature allows your customers to securely access, verify, and update their own contact information and consent preferences. This not only improves data accuracy but also builds trust and empowers customers, a critical differentiator that supports the best possible customer data records in an increasingly challenging financial services sector.
A Focus on Data Accuracy, Not Just Analytics – While we enable richer analytics, our primary focus is on ensuring a reliable single source of truth. So, we prioritize data accuracy and governance above all else. This is a critical distinction from CDPs, or even CRMs which are optimized for marketing segmentation and campaign management or customer service and support request management. For financial institutions, a reliable data foundation is paramount for risk management, compliance, and operational integrity—the core functions of the business.
Seamless Integration, Unmatched Flexibility – Data models in Pretectum are adaptable, and can accommodate the complex, multi-layered relationships inherent in financial services; from individual customers and joint accounts to corporate entities and their authorized representatives. The rich search and integration APIs ensure seamless connectivity, allowing you to easily embed trusted customer data into every touchpoint, from the call center to your mobile banking app.
A Pretectum Path Forward
The era of fragmented customer data is a luxury financial institutions cannot afford. The cost of manual reconciliation, compliance risk, and a subpar customer experience is too high. The path forward isn’t to build another siloed solution but to create a single, unified view of your customer that acts as a central nervous system for your entire organization.
Pretectum CMDM is not just a technology platform; it serves as a gateway technology to a strategic shift. Pretectum is about re-establishing trust by ensuring that every interaction, every decision, and every compliance report is based on a single, accurate, and real-time understanding of your customer.
In our modern world of increasing complexity and risk, and highly competitive customer loyalty stakes, this is the definitive way to protect the longevity of your institution and unlock the true value of customer data.
#LoyaltyIsUpForGrabs


